Liquid-based cervical cytology and Human Papillomavirus (HPV) genotyping for cervical cancer screening and prevention
By: Laura Tapia Vela* Jairo A. Mesa Arango**
*Head of Anatomic Pathology, Medical Pathologist, Laboratorio Clínico Hematológico S.A. Medellín, Colombia.
** Molecular Laboratory Scientist, PhDc in Biology, Laboratorio Clínico Hematológico S.A. Medellín, Colombia.
Published on 07/23/2021
Cervical cancer is a major public health problem, accounting for approximately 530,000 new cases per year and the vast majority of cancers attributable to human papillomavirus (HPV) worldwide. In figures published for 2012, 528,000 new cases of the disease and 266,000 deaths were reported, occurring, for the most part (~90%), in women with low or middle income and from developing countries, with problems of access to timely diagnostic and treatment services. In developed countries, cervical cancer rates reported for the last three decades show a significant decrease, largely as a result of screening programs that allow early detection and appropriate management of the disease.
In Latin America, cervical cancer is the second leading cause of cancer in women, after breast cancer. In Colombia, the behavior is similar, which has led to the adoption of measures for the reduction of cervical cancer, such as screening women over 30 years of age with the DNA detection test for high-risk HPV and the performance of cervical cytology as a triage method in patients with positive HPV test. Likewise, with preventive vaccination programs against infection by high-risk HPV genotypes. Despite the progressive decrease in the number of reported cases of the disease, for 2018, 3,853 new cases and an estimated 1,775 deaths were reported, indicating the persistence of the disease in our environment.
Cervical cytology and HPV DNA testing are currently considered the most efficient screening methods for the detection of premalignant cervical lesions and high-risk HPV genotypes, respectively, both being early intervention tools aimed at reducing the incidence and mortality rate due to cervical cancer. Therefore, screening algorithms based on these tests, as well as standardized follow-up and treatment protocols, have been established in health systems around the world in order to achieve high coverage.

Accordingly, national health policies in our country prioritize actions aimed at the promotion of protective factors, the promotion of self-care through the performance of cervical cytology in high quality health services, continuity in the diagnostic and/or treatment processes, and strict follow-up by trained medical personnel.
Human Papillomavirus (HPV)
To date, five HPV genera, 49 species and more than 200 genotypes have been described, classified based on differences in the sequence of the gene coding for the L1 protein, the main structural protein of the viral capsid. According to the ability to produce persistent infections, leading to the development of premalignant or malignant lesions, the different HPV types are grouped into low-risk and high-risk genotypes; the latter identified by the numbers 16, 18, 31, 33, 35, 35, 39, 45, 51, 52, 56, 56, 58, 59, 66 and 68.
Most of the known genotypes are considered low risk, since they are associated with mainly transient and limited infections, visualized as low-grade squamous intraepithelial lesions, which are mostly controlled by the immune system. On the other hand, high-risk genotypes are related to the induction of high-grade squamous intraepithelial lesions, which, if left untreated, can evolve into cervical cancer after 10 to 20 years, which is why they are considered the real agents involved in the development of cervical premalignant lesions and cervical cancer.
The two high-risk HPV genotypes that have been primarily associated with premalignant lesions and cervical carcinoma are 16 and 18. For example, positivity for HPV 16 ranges from 20% to 28% of Pap smears reported as normal or with low-grade squamous intraepithelial lesions, and increases to 47% to 63% of Pap smears reporting high-grade squamous intraepithelial lesions.
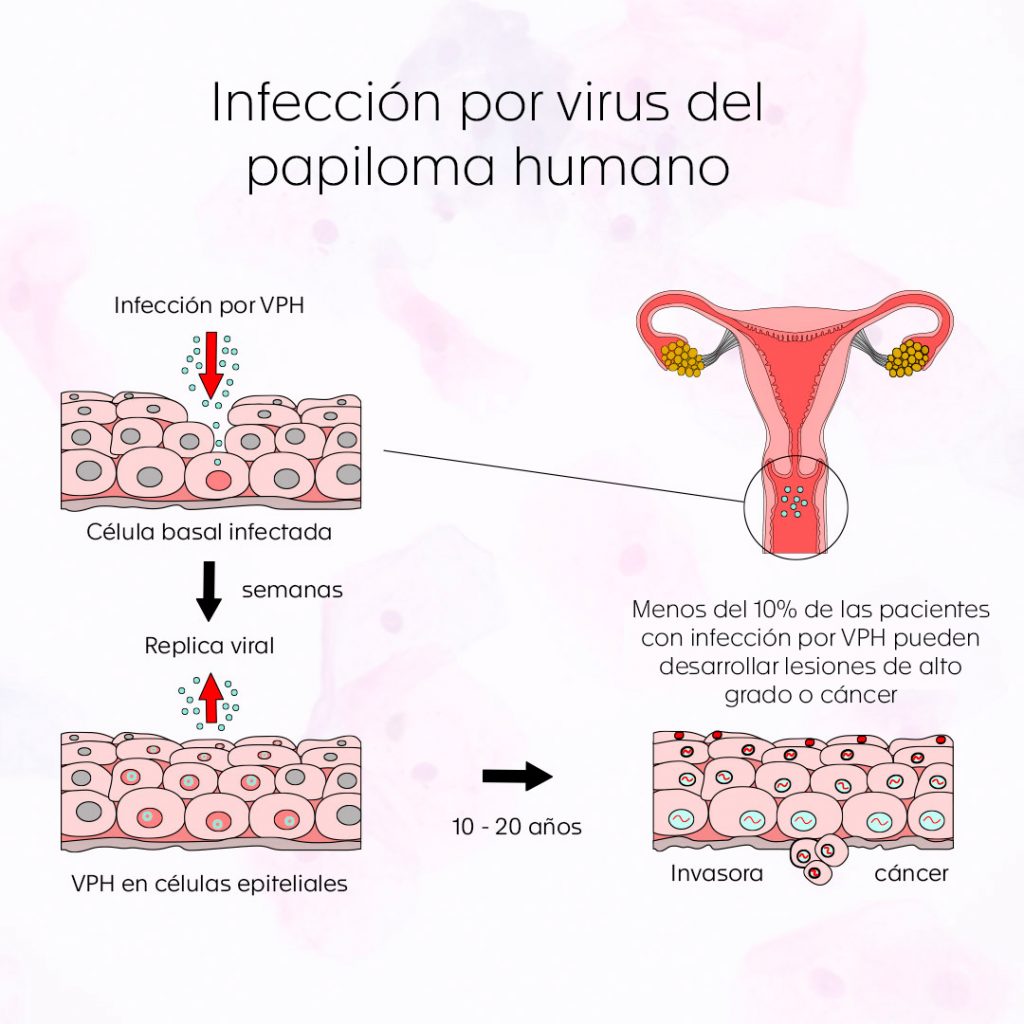
Worldwide, it is estimated that 75% of sexually active people will be infected with HPV at least once in their lifetime. In developing countries, the prevalence of infection is estimated to be close to 14%, higher than that reported in developed countries (10%). However, as mentioned above, most HPV infections (90%) are transient and resolve spontaneously within the first year or two after infection (5). Clearance of infections is closely related to the immune status, genetic predisposition and hormonal levels of the infected person, as well as reinfection or coinfection with another genotype of the virus. Infections caused by high-risk viral genotypes usually resolve within 4 to 20 months and those caused by low-risk genotypes regress more rapidly.
HPV and cervical carcinoma:
There are two mechanisms of action of HPV on squamous epithelia of the skin and mucous membranes. The first corresponds to transient infection and viral replication in cells, utilizing the cellular machinery to generate more viral particles. This interaction is typically seen as a low-grade squamous intraepithelial lesion, formerly called mild dysplasia or grade 1 intraepithelial neoplasia (CIN1). The second form of interaction corresponds to premalignant or precancerous lesions, which are characterized by a mismatch between viral proliferation and epithelial cell differentiation. It is to say, there is an overexpression of the virus that originates from the rapid growth and uncontrolled epithelial cells undifferentiated and that, morphologically, is observed as a high grade squamous intraepithelial lesion, previously called intraepithelial neoplasia grade 2 (CIN 2) or intraepithelial neoplasia grade 3 (CIN 3) .
The main objective of the screening by cervicouterine cytology is the detection and timely treatment of premalignant lesions, in order to decrease the appearance of invasive carcinoma of the uterine cervix, given up to 30% to 50% of the high-grade lesions can progress in the following 30 years to this type of cancer.
Due to morbidity and mortality derived from cervical cancer development, it is of great relevance to obtain information from the HPV genotypes of high risk present in the periods of infection and persistence, using methods that allow us to perform a reliable molecular screening, diagnosis and typing, as a strategy for early detection and, consequently, the reduction of mortality associated with this cancer.
Screening tests:
The goal of current screening methods is to detect high-grade cervical lesions and non-invasive cervical carcinomas for which successful treatment exists and thus prevent progression to invasive cervical carcinoma. Current options include cervical cytology and molecular testing for HPV DNA.
Conventional cervicovaginal cytology:
Conventional cervical cytology or Papanicolaou (PAP), developed by George Papanicolaou, is considered the most cost-effective screening method to detect premalignant cervical lesions early and prevent progression to malignant lesions. For this, a sample of cells is taken from the cervix with a spatula and cytobrush, which is manually spread on a slide and fixed for subsequent staining and microscopic study.
The Bethesda system is the standardized guide for the evaluation of cervical cytology worldwide, provides the criteria related to the quality of the sample and establishes a report with unified criteria. In this regard, it is critical to understand that the terminology reflects the current understanding of the biology of HPV involvement of the squamous epithelium, either as a viral infection or as a premalignant virus-associated lesion.

Liquid-based cervical cytology:
In May 1996, liquid-based cytology was approved by the U.S. Food and Drug Administration (FDA). This decision was made based on studies that showed a significant increase in the detection of low-grade squamous intraepithelial lesions or more advanced lesions, compared to conventional cytology. In addition, its use showed a reduction in the number of suboptimal or inadequate samples, and avoided the repetition of smears.
This technique uses the same process as the conventional one for taking the sample of cervical cells, but unlike this, the sample is introduced in a container with a conservative medium, from which the slides are prepared for microscopic analysis. This solves some of the problems of the conventional Papanicolaou technique, such as poor capture of the entire sample, insufficient fixation, random distribution of abnormal cells, existence of disturbing elements and poor quality of the smear. It also facilitates reading by eliminating blood or other artifacts, which increases the number of satisfactory samples for evaluation and helps to better detect intraepithelial lesions. Finally, it allows molecular tests for the detection of HPV and immunohistochemistry to be performed jointly and using the same sample.

Molecular diagnosis of HPV:
The causal relationship between the development of cervical cancer and infection with high-risk HPV genotypes has led to the development of strategies for their detection and genotypic characterization, as a cervical cancer prevention measure. Since the presence of HPV cannot be determined by morphological findings on cytology, detection of specific HPV antibodies (serological tests) or specific clinical criteria, its detection and genotyping relies on the use of molecular tests.
Molecular tests for HPV are mostly aimed at detecting DNA of high-risk genotypes and are used as diagnostic strategies, classification of low-grade cytological abnormalities (according to the risk associated with the type of HPV identified), evaluation of persistent infections, post-treatment follow-up of a high-grade squamous intraepithelial lesion and epidemiological surveillance in public health at the national and international level.
The high negative predictive value, together with its high sensitivity, prompted the use of HPV DNA detection techniques as primary tests for cervical cancer screening and, in combination with cervical cytology findings, to stratify the risk of severe cervical disease and guide the selection of appropriate treatments according to the cervical lesion found and the follow-up required. The use of cytology and HPV DNA testing in a combined approach increases the sensitivity of cervical screening (up to 90%) and significantly reduces the risk of premalignant cervical lesions over a 5-year period (considered from a negative cervical cytology result).
There are a number of useful molecular tests for the detection of HPV DNA or its genotyping directly in cytology specimens. Poljak et al. reported a total of 254 different commercial tests available for HPV detection by 2020. These tests, which differ from each other in the number and type of HPV they can detect, their clinical sensitivity and specificity, and the type of technology they use for detection, can be classified mainly into two:
- Tests for direct detection of viral DNA: based on hybridization techniques, including some in disuse such as in situ hybridization, southern blot and dot blot, or signal amplification of nucleic acids such as hybrid capture tests.
- Amplification tests of specific regions of viral DNA: most of them based on conventional polymerase chain reaction (PCR) or real-time (RT-PCR) techniques for the amplification of specific regions of HPV DNA, including the L1 (late gene coding for structural proteins that make up the HPV nucleocapsid), E6 and E7 (early non-structural genes) genes.
The molecular test considered to be the gold standard for HPV detection is hybrid capture (DNA-RNA). There are two commercial tests based on hybrid capture and approved by the FDA for HPV detection. These allow DNA detection of up to 14 high-risk HPV types (16, 18, 31, 33, 35, 35, 39, 45, 51, 52, 56, 58, 59, and 68) and up to 5 low-risk genotypes (6, 11, 42, 43, and 44). Despite including HPV genotypes considered clinically relevant, their execution is laborious and limited only to the detection (positive/negative) of HPV, grouped as high-risk or low-risk genotypes, without allowing their individual and specific genotyping, which is of great relevance in the follow-up of persistent infections, the monitoring of vaccination programs and in epidemiological surveillance programs and studies.
Hybrid capture tests have been shown to be useful in the routine diagnosis of HPV; however, there are many alternative molecular tests, with better clinical sensitivity and specificity, and less laborious, which allow detection, genotyping and quantification of HPV in a single assay. These tests are mostly based on the amplification of specific regions of HPV DNA.
PCR- and RT-PCR-based viral DNA amplification tests, mostly designed for L1 gene amplification, are of better sensitivity and specificity than hybrid capture, and allow simultaneous genotyping and quantification of multiple HPV genotypes. In addition to allowing individual characterization of clinically relevant genotypes (high-risk genotypes), DNA amplification tests have been coupled with automated technologies and platforms to reduce the cost and time to process cytology samples and obtain results.
AnyplexTM II is a multiplex real-time PCR (RT-PCR) test that allows qualitative (Positive/Negative) and simultaneous detection of 14 HPV genotypes. This RT-PCR test allows amplification of a specific region of the L1 gene of the virus, detection (using fluorescently labeled probes) and differentiation (genotype discrimination) of the DNA of HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51 52, 56, 58, 59, 66, and 68, classified as high risk for cervical cancer.
In addition to being a robust test with high sensitivity and specificity, the Anyplex II RT-PCR test was evaluated and clinically validated under international guidelines and standards for cervical cancer screening and diagnosis. This test can be applied directly to liquid-based cytology samples and is considered a primary screening method to identify women at high risk for the development of cervical cancer or the presence of high-risk disease. It can also be used to evaluate cytology samples from patients with atypical cervical cytology results such as ASCUS (squamous cell atypia of undetermined significance), in order to determine the need for colposcopy referral or to support molecular diagnosis of HPV infections.
Why choose the Laboratorio Clínico Hematológico:
At the Hematológico we care about providing you with the best screening strategies to prevent cervical cancer. Currently, we offer, in all our locations, alternatives for access to liquid-based cervical cytology, which can be accompanied jointly with the AnyplexTM II RT-PCR test for the detection and genotyping of high-risk HPV DNA, using a single cytological sample.
We also have expert staff with the best scientific and human quality, which guarantees the analysis and integration of the results of both tests for personalized counseling.
Our technological infrastructure and personnel allow us to offer fast and reliable results according to your specific needs. Additionally, we are promoting agreements so that you can access cytology screening and HPV DNA detection from allied gynecologists’ offices, for greater convenience and easy access to our services.
Bibliography
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type: Worldwide burden of cancer attributable to HPV. Int J Cancer. 15 de agosto de 2017;141(4):664-70.
- World Health Organization. Comprehensive Cervical Cancer Control. A guide to essential practice. Geneva; WHO 2006. World Health Organ Compr Cerv Cancer Control Guide Essent Pract Geneva WHO. 2006;180-200.
- República de Colombia, Ministerio de Salud y Protección Social. Guía de Práctica Clínica para la detección y manejo de lesiones precancerosas de cuello uterino. Guía para pacientes y cuidadores. Colombia 2014. Guías de Práctica Clínica. 2014. 1 p.
- Almonte M, Murillo R, Sánchez GI, Jerónimo J, Salmerón J, Ferreccio C, et al. Nuevos paradigmas y desafíos en la prevención y control del cáncer de cuello uterino en América Latina. Salud Publica Mex. 2010;52(6):544-59.
- Asiaf A, Ahmad ST, Mohammad SO, Zargar MA. Review of the current knowledge on the epidemiology, pathogenesis, and prevention of human papillomavirus infection. Eur J Cancer Prev. 2014;23(3):206-24.
- Bruni L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, et al. Human Papillomavirus and Related Diseases in the World. Barcelona, España: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre); 2019.
- Muñoz N, Bravo LE. Epidemiology of cervical cancer in Colombia. Salud Publica Mex. 2014;56(5):431-9.
- Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015;123(5):271-81.
- Bzhalava D, Eklund C, Dillner J. International standardization and classification of human papillomavirus types. Virology. 2015;476:341-4.
- Leal SM, Gulley ML. Current and Emerging Molecular Tests for Human Papillomavirus–Related Neoplasia in the Genomic Era. J Mol Diagn. 2017;19(3):366-77.
- Granberg S, Gjelland K, Ekerhovd E. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2009;23(5):667-78.
- Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Ronald D, et al. The Lower Anogenital Squamous Terminology Standardization Project for HPV-Associated Lesions: Background and Consensus Recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. 2012;136(October).
- Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011;103(5):368-83.
- Wentzensen N, Schiffman M, Palmer T, Arbyn M. Triage of HPV positive women in cervical cancer screening. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2015/11/28 ed. marzo de 2016;76 Suppl 1(Suppl 1):S49-55.
- Gibb RK, Martens MG. The impact of liquid-based cytology in decreasing the incidence of cervical cancer. Rev Obstet Gynecol. 2011;4(Suppl 1):S2-11.
- Papanicolaou GN, Traut HF. The Diagnostic Value of Vaginal Smears in Carcinoma of the Uterus. Am J Obstet Gynecol. 1 de agosto de 1941;42(2):193-206.
- Nayar R, Wilbur DC. The bethesda system for reporting cervical cytology: Definitions, criteria, and explanatory notes. Bethesda Syst Report Cerv Cytol Defin Criteria Explan Notes. octubre de 2015;35(10):1-321.
- Nayar R, Goulart RA, Davey DD. Primary HPV cervical cancer screening in the United States: Are we ready? J Am Soc Cytopathol. febrero de 2018;7(1):50-5.
- Gradíssimo A, Burk RD. Molecular tests potentially improving HPV screening and genotyping for cervical cancer prevention. Expert Rev Mol Diagn. 2017;17(4):379-91.
- Groves IJ, Coleman N. Pathogenesis of human papillomavirus-associated mucosal disease. J Pathol. 2015;235(4):527-38.
- Poljak M, Oštrbenk Valenčak A, Gimpelj Domjanič G, Xu L, Arbyn M. Commercially available molecular tests for human papillomaviruses: a global overview. Clin Microbiol Infect. 2020;26(9):1144-50.
- Abreu ALP, Souza RP, Gimenes F, Consolaro MEL. A review of methods for detect human Papillomavirus infection. Virol J. 2012;9(1):1-9.
- Hwang SJ, Shroyer KR. Biomarkers of cervical dysplasia and carcinoma. J Oncol. 2012;2012.
- Hesselink AT, Sahli R, Berkhof J, Snijders PJF, van der Salm ML, Agard D, et al. Clinical validation of AnyplexTM II HPV HR Detection according to the guidelines for HPV test requirements for cervical cancer screening. J Clin Virol. marzo de 2016;76(3):36-9.
- Jung S, Lee B, Lee KN, Kim Y, Oh EJ. Clinical validation of Anyplex II HPV HR detection test for cervical cancer screening in Korea. Arch Pathol Lab Med. 2016;140(3):276-80.



