Hemophilia: from a disease of kings to an orphan disease
Compartir
Hemophilia includes a group of X-linked inherited diseases caused by mutations in the genes for coagulation factors FVIII (hemophilia A) and FIX (hemophilia B) and associated with bleeding. Affected patients may have mild, moderate or severe forms of the disease, depending on the plasma level of each factor. Despite being well recognized by the general public, it is considered an orphan disease, with a prevalence of 1 in 5,000 live male births in the case of hemophilia A and 1 in 30,000 in the case of hemophilia B.
The peculiarities of hemophilia have been recognized since ancient times. The Talmud indicated that male infants should not be circumcised if two brothers had died from bleeding associated with the procedure. A 12th century description by the Arab physician Albucasis describes a family whose male members die from bleeding after minor injuries. In modern times, the physician John Conrad Otto, of Philadelphia-USA, published in 1803 the “Report of the hemorrhagic disposition existing in certain families”; however, the term “hemophilia” was first used in 1828, in a paper by Hopff of the University of Zurich. Hemophilia has been called “the disease of kings”, since numerous members of European royal houses of the time suffered from it, due to the fact that Queen Victoria of England was a carrier of hemophilia B, as were her daughters Alice and Beatrice, who transmitted the disease to the Spanish, Russian and German royal houses; in addition, her son Leopold suffered from frequent hemorrhages and died at the age of 31 from a cerebral hemorrhage.
Initially, it was thought that the hemorrhagic tendency of hemophilia was due to fragility of the blood vessels; in the 1930s, it was proposed that it was associated with alterations in the platelets. Subsequently, in 1937, it was found that by adding a substance, which at that time was called “antihemophilic globulin”, to the patient’s blood, the clotting defect was corrected. In 1944, Pavlosky’s observations showed that the blood of one hemophilia patient corrected the clotting defect of another patient, and vice versa, demonstrating the defect in two different clotting factors, FVIII and FIX. These findings allowed an accurate diagnosis and the development of an appropriate treatment for this disease.
Etiology and pathophysiology
To maintain hemostasis, the human body has a complex system that allows the balance of procoagulant, anticoagulant and fibrinolytic states to maintain blood fluidity within the vascular system and to achieve rapid thrombus development in response to vascular injury. In this process, the sequential activation of complexes composed of vitamin K-dependent factors (FVII, FIX, FX) and their respective cofactors (tissue factor, FVIII, FV) is essential. FVIII and FIX factors are part of the intrinsic coagulation pathway (see figure 1).
Clinical features
The clinical presentation of hemophilia A and B is similar, with the joints being the main site of spontaneous bleeding, mainly the ankle, followed by the elbows and knees. Approximately half of children with severe hemophilia develop intramuscular hematomas as early as 6 to 8 months of age.
Patients with mild hemophilia usually bleed excessively only in association with surgery or major trauma; however, those with severe hemophilia have frequent episodes of spontaneous bleeding, especially intra-articular or muscular, following minor trauma.
Recurrent joint bleeding induces a cascade of inflammatory and degenerative processes that injure the synovium, cartilage and bone. The major trigger of these processes is iron released into the synovial fluid, which has proinflammatory and angiogenic activity; the associated neovascularization leads to the formation of new friable vessels, more prone to bleeding, leading to a cycle of bleeding, iron accumulation, synovial hypertrophy and rebleeding.
Due to these cycles, many patients develop chronic synovitis with joint edema; others reach hemophilic arthropathy with severe osteochondral damage. This arthropathy leads to chronic pain, loss of range of motion, muscle atrophy and, because of this, reduced quality of life (8). Another frequent complication is intracranial hemorrhage, with an incidence of 1.9% and a mortality rate of 19.6%.
Diagnosis and follow-up
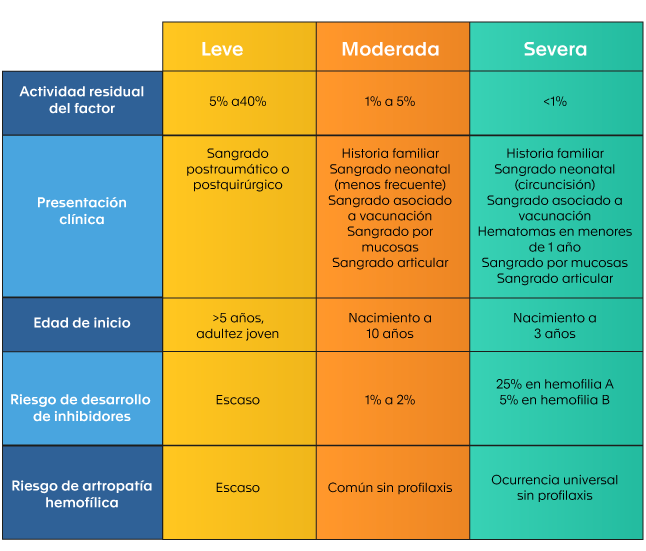
Table 1. Classification of hemophilia according to clinical presentation
The formation of these inhibitors is a complex and multifactorial process; it has been suggested in some studies that it may vary according to the type of product used in the treatment (plasma-derived FVIII versus recombinant FVIII); however, the evidence is inconclusive.
Inhibitors are high-affinity polyclonal IgG antibodies directed against the FVIII protein and can be of two types: type 1, which completely inactivate FVIII, and type 2, which incompletely inactivate FVIII. Patients with high antibody titers develop severe bleeding complications that do not respond to clotting factor replacement. Although many of these inhibitors are transient, or resolve with immunotherapy, up to 15% of patients with hemophilia A and 3% of those with hemophilia B have persistent antibodies.
Treatment: historical evolution
In the 1950s and 1960s, patients with hemophilia were treated with fresh blood or plasma only; however, because the low concentration of clotting factors required in these products was not sufficient to treat severe bleeds, a large number of patients died in infancy or young adulthood.
In 1964, it was discovered that the cryoprecipitated fraction of plasma contains large amounts of FVIII. This made it possible to concentrate enough of the factor in smaller volumes and to perform larger surgeries. However, the modern era of hemophilia treatment began in the 1970s with the production of lyophilized clotting factor concentrates. This advance made it possible to improve the quality and prolong the life expectancy of patients due to the implementation and dissemination of home replacement therapy, early control of hemorrhages and the reduction of musculoskeletal damage associated with inadequate treatment.
Primary prophylaxis was able to prevent most episodes of spontaneous joint bleeding, reducing arthropathy. On the other hand, the discovery of desmopressin, which shortens the prolonged activated partial thromboplastin time and bleeding time, favoring the elevation of FVIII and von Willebrand factor, has become an economical and safe treatment for patients with mild hemophilia A.
Unfortunately, the era of plasma concentrates for the treatment of hemophilia suffered a major setback due to the transmission of human immunodeficiency virus (HIV) and Hepatitis C virus (HCV) through clotting factor concentrates made from plasma from multiple donors. Thousands of hemophilia patients died from complications of HIV/AIDS in the 1980s and 1990s. As a consequence, safer treatments were sought and viral inactivation techniques were implemented in the production of plasma-derived factor concentrates and viral screening in donated blood, all of which increased the safety of these derivatives.

The major breakthrough in the treatment of hemophilia occurred in the last decades of the 20th century and was related to the progress of recombinant DNA technology, which allowed the development of recombinant FVIII and FIX and thus decreased the risk of pathogen transmission. As a result of progress in therapy for the hemophilias, the life expectancy of patients has caught up with that of the general population. This has led to the development of age-associated chronic diseases previously unseen in the hemophilia population.
Recent therapeutic approaches include specific antibodies that mimic the coagulant function of FVIII, inhibition of anticoagulant proteins, such as antithrombin, with RNA interference molecules, and the tissue factor inhibitory pathway with monoclonal antibodies.
On the other hand, phase 3 studies of gene therapy are underway as a curative option against hemophilia. The goal of gene therapy is that patients do not require coagulation factor replacement therapy and do not bleed. Successful gene therapy results in endogenous expression of clotting factor, reaching a stable level and with a sustained duration of action. This would make prophylaxis and intravenous infusions unnecessary. In addition, it has been postulated that endogenous expression of the factors could be less immunogenic, decreasing or avoiding the generation of inhibitors.
In the future, it is expected to have an increase in the production of FVIII and FIX concentrates to supply developing countries; in addition, the processing of molecules with longer half-life and lower immunogenicity is expected.
World Hemophilia Day
The World Federation of Hemophilia (WFH), since 1989, commemorates every year on April 17 the World Hemophilia Day, in honor of the birthday of its founder, Frank Schnabel. The aim of this day is to bring the community with bleeding disorders closer together and unite them. For this purpose, hemophilia patient organizations and specialized treatment centers carry out academic and sports activities, exhibitions and other events to raise awareness in the community about hemophilia, its complications and implications in the lives of patients with hemophilia.
In 2021, the WFH took as its motto for this day “ADAPTING TO CHANGE: PRESERVING CARE IN A NEW WORLD”, due to the strong impact that the COVID-19 pandemic has had on people with bleeding disorders and the global changes it has implied. The WFH remains committed to ensuring access to appropriate and sustainable treatment and care for all people with bleeding disorders, adapting to the changes made necessary by the pandemic.
Hemophilia in Colombia
By 2017, 2170 people with hemophilia were registered in Colombia, of whom 1794 had hemophilia A and 376 had hemophilia B, and 1167 were classified as severe hemophilia. Of these patients, 1284 were on prophylaxis and 324 (14.9% of the total) had inhibitors. During the same period, 16 patients with hemophilia died; however, only two of them died due to complications associated with the disease.
Recently, the National Registry of Hemophilia and other coagulopathies was published as a multisectoral initiative to centralize sociodemographic, clinical and economic information on these patients, by means of a consensus of experts, in order to monitor morbidity and mortality, evaluate access to health services, their impact on the complications of the disease and the costs associated with medical care. This registry is expected to guide rational decision making for an efficient use of economic resources, as well as to promote health research to improve the quality of life and reduce the associated disabilities in patients with hemophilia.
In the Laboratorio Clínico Hematológico
The Hematológico offers tests for the evaluation of hemostasis divided into two areas following the physiological order: platelet aggregation and coagulation studies specific to primary hemostasis and studies associated with factors and dynamics of secondary hemostasis. These tests are essential in the study of patients with abnormal bleeding conditions such as hemophilia or von Willebrand’s disease. Within the specialized tests for the diagnosis of bleeding disorders, we offer the determination of coagulation factors VIII, IX and XI, for the diagnosis of deficiencies and as a follow-up of factor VIII and IX replacement therapy.
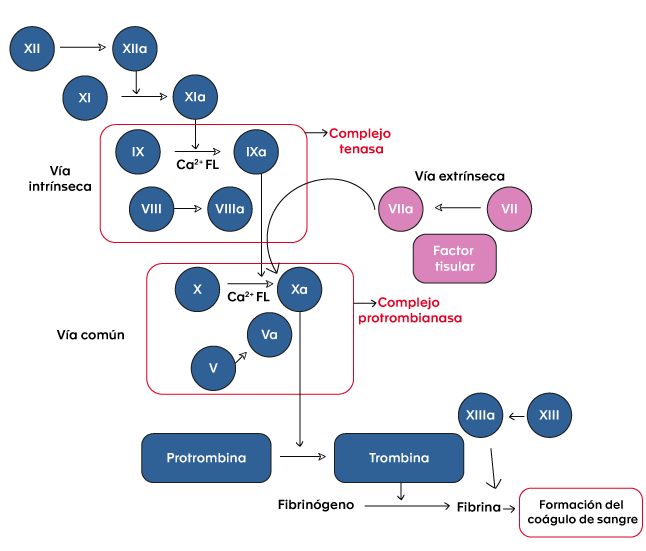
Figure 1. Coagulation cascade.
Bibliography
- Franchini M. The modern treatment of haemophilia: a narrative review. Blood Transfus. abril de 2013;11(2):178-82.
- Franchini M, Mannucci P. Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis. 2012;7(1):24.
- Biggs R. CHRISTMAS DISEASE. A CONDITION PREVIOUSLY MISTAKEN FOR HAEMOPHILIA. British Medical Journal. 27 de diciembre de 1952;1378-82.
- Kizilocak H, Young G. Diagnosis and Treatment of Hemophilia. Clin Adv Hematol Oncol. 2019;17(6):8.
- Van Den Berg HM, De Groot PHG, Fischer K. Phenotypic heterogeneity in severe hemophilia: Prothrombotic risk factors and phenotype of severe hemophilia. J Thromb Haemost. 9 de julio de 2007;5:151-6.
- Hoyer LW. Hemophilia A. N Engl J Med. 6 de enero de 1994;330(1):38-47.
- Acharya SS, Kaplan RN, Macdonald D, Fabiyi OT, DiMichele D, Lyden D. Neoangiogenesis contributes to the development of hemophilic synovitis. Blood. 24 de febrero de 2011;117(8):2484-93.
- Jansen NWD, Roosendaal G, Lafeber FPJG. Understanding haemophilic arthropathy: an exploration of current open issues. Br J Haematol. 2008;143(5):632-40.
- Witmer C, Presley R, Kulkarni R, Michael Soucie J, Manno CS, Raffini L. Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States: Intracranial Haemorrhage in Haemophilia. Br J Haematol. enero de 2011;152(2):211-6.
- Attard C, van der Straaten T, Karlaftis V, Monagle P, Ignjatovic V. Developmental hemostasis: age-specific differences in the levels of hemostatic proteins. J Thromb Haemost. octubre de 2013;11(10):1850-4.
- Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. noviembre de 2014;12(11):1935-9.
- Gouw SC, van den Berg HM, Oldenburg J, Astermark J, de Groot PG, Margaglione M, et al. F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood. 22 de marzo de 2012;119(12):2922-34.
- Bagnall RD, Waseem N, Green PM, Giannelli F. Recurrent inversion breaking intron 1 of the factor VIII gene is a frequent cause of severe hemophilia A. Blood. 1 de enero de 2002;99(1):168-74.
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. The Lancet. julio de 2016;388(10040):187-97.
- Cannavò A, Valsecchi C, Garagiola I, Palla R, Mannucci PM, Rosendaal FR, et al. Nonneutralizing antibodies against factor VIII and risk of inhibitor development in severe hemophilia A. Blood. 9 de marzo de 2017;129(10):1245-50.
- Gringeri A, Mantovani LG, Scalone L, Mannucci PM, the COCIS Study Group. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 1 de octubre de 2003;102(7):2358-63.
- Mannucci PM. Back to the future: a recent history of haemophilia treatment. Haemophilia. julio de 2008;14(s3):10-8.
- Mannucci PM, Tuddenham EGD. The Hemophilias — From Royal Genes to Gene Therapy. N Engl J Med. 2001;7.
- Pipe S. Recombinant clotting factors. Thromb Haemost. 2008;99(11):840-50.
- Shima M, Hanabusa H, Taki M, Matsushita T, Sato T, Fukutake K, et al. Factor VIII–Mimetic Function of Humanized Bispecific Antibody in Hemophilia A. N Engl J Med. 26 de mayo de 2016;374(21):2044-53.
- Petersen LC. Hemostatic properties of a TFPI antibody. Thromb Res. mayo de 2012;129:S44-5.
- Shapiro AD, Angchaisuksiri P, Astermark J, Benson G, Castaman G, Chowdary P, et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood. 28 de noviembre de 2019;134(22):1973-82.
- Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. febrero de 2018;65(2):e26865.
- 17 de abril – Día Mundial de la Hemofilia > Pfizer.es [Internet]. [citado 31 de marzo de 2021]. Disponible en: https://www.pfizer.es/salud/dias_salud/17_abril_dia_mundial_hemofilia.html
- Día mundial de la hemofilia 2021 – World Federation of Hemophilia [Internet]. [citado 31 de marzo de 2021]. Disponible en: https://www.wfh.org/es/eventos/dia-mundial-de-la-hemofilia
- Liga Colombiana de Hemofílicos y otras deficiencias sanguíneas [Internet]. [citado 2 de marzo de 2021]. Disponible en: http://colhemofilicos.org.co/
- Acuña L. Situación de la hemofilia en Colombia 2017.pdf [Internet]. Bogotá; 2018. 176 p. Disponible en: http://colhemofilicos.org.co/_assets/archives/presentaciones/Libro_situacion_hemofilia_en%20Colombia_2017.pdf
- Alvis LF, Sánchez P, Acuña L, Escobar G, Linares A, Solano MH, et al. National registry of haemophilia and other coagulopathies: A multisector initiative in the Colombian Health System. Haemophilia [Internet]. noviembre de 2020 [citado 24 de marzo de 2021];26(6). Disponible en: https://onlinelibrary.wiley.com/doi/10.1111/hae.14138



